

Watch Just How Small is an Atom? (5:27 min) (credit middle: modification of work by babyknight, CC BY 2.0 credit right: modification of work by Paxson Woelber, CC BY 2.0) Figure 5.6a Perspective about an Atoms Relative Size: If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry. For a perspective about their relative sizes, consider this: If the nucleus were the size of a blueberry, the atom would be about the size of a football stadium (Figure 5.6a). The diameter of an atom is on the order of 10 −10 m, whereas the diameter of the nucleus is roughly 10 −15 m-about 100,000 times smaller. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The development of modern atomic theory revealed much about the inner structure of atoms. Calculate average atomic mass and isotopic abundance.Define the atomic mass unit and average atomic mass.Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others.By the end of this section, you will be able to:
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants.įrom providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation. Physics Wallah's main focus is to make the learning experience as economical as possible for all students. We believe in empowering every single student who couldn’t dream of a good career in engineering and medical field earlier. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We successfully provide students with intensive courses by India's top faculties and personal mentors.
Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app. We also provide extensive NCERT solutions, sample papers, NEET, JEE Mains, BITSAT previous year papers, which makes us a one-stop solution for all resources. Physics Wallah is India's top online ed-tech platform that provides affordable and comprehensive learning experience to students of classes 6 to 12 and those preparing for JEE and NEET exams.
#Equation for atomic mass number pdf#
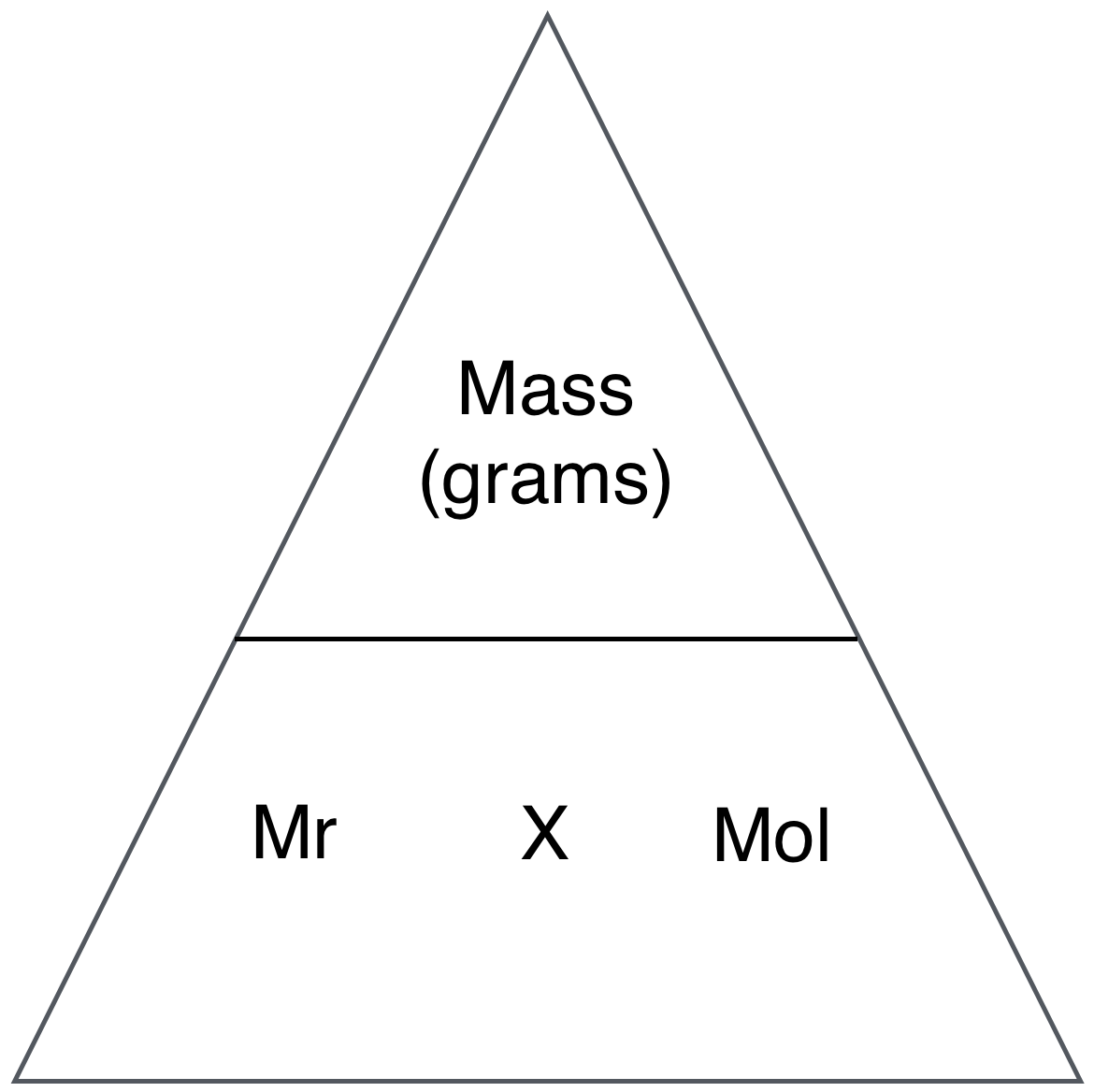
For more chemistry Formulas visit formula section.įind the list of Atomic Mass of Elements in the below Pdf So it can be an integer or fractional value. While atomic number is the average of atomic weights of all the isotopes of an element present in the nature. It is nearly equal to the atomic mass of that element.Hence, for an atom mass number = number of protons + number of neutrons.ĭifference between Mass Number and Atomic Mass:Ī mass number is always a whole number whereas atomic mass is usually not a whole number because mass number is the sum of two integers so it can never be fractional, else we can say number of protons and neutrons can never be fractional. collectively known as nucleons) present in the nucleus of an element is called mass number and is denoted by A. The sum of the number of protons and neutrons (i.e. Mass Number (A): Proposed after the discovery of neutron. Hence, atomic number of an element = total number of protons present in an atom. The number of protons present in an atom is called the atomic number which is, denoted by Z.


 0 kommentar(er)
0 kommentar(er)
